Abstract
Introduction: Immune thrombocytopenia (ITP) is an autoimmune disease associated with autoantibody-mediated platelet destruction and impaired platelet production, resulting in thrombocytopenia and high bleeding risk. Fatigue and impairment of both memory and concentration are also common in ITP, theoretically due to acute neural inflammation, microbleeding, and thrombotic occlusions of small blood vessels in the brain. However, little is known about the nature and severity of cognitive impairment in patients with chronic ITP. Tests from the Cogstate Brief Battery (CBB) and composite scores computed from these have a high sensitivity to cognitive dysfunction in clinical and clinical trial contexts. The aim of this analysis was to assess cognitive impairment among difficult-to-treat patients with ITP.
Methods: Participants (N=60) in the ongoing, global phase 1/2 study (NCT03395210) assessing the safety and efficacy of the Bruton tyrosine kinase inhibitor rilzabrutinib in ITP, were eligible for cognitive testing at baseline. In the CBB, the Detection test (DET) measured psychomotor function, the Identification test (IDN) measured attention, the One Card Learning test (OCL) measured visual learning, and the One Back test (ONB) measured working memory. For each participant, prior to rilzabrutinib treatment, the score on each test was compared with age-matched normative data to produce a standardized score (Z-score). Z-scores measure the distance between an observed value and a reference mean using standard deviation units and can be positive or negative, indicating that the observed value lies above or below the mean, respectively. Z-scores ≤-1 denoted impairment. Descriptive summary statistics, including mean Z-scores for individual tests and composite cognitive scores (Psychomotor/Attention, Learning/Memory), and level of impairment are presented. Relationships between cognitive impairment and demographics, concomitant medication, and medical history were also explored.
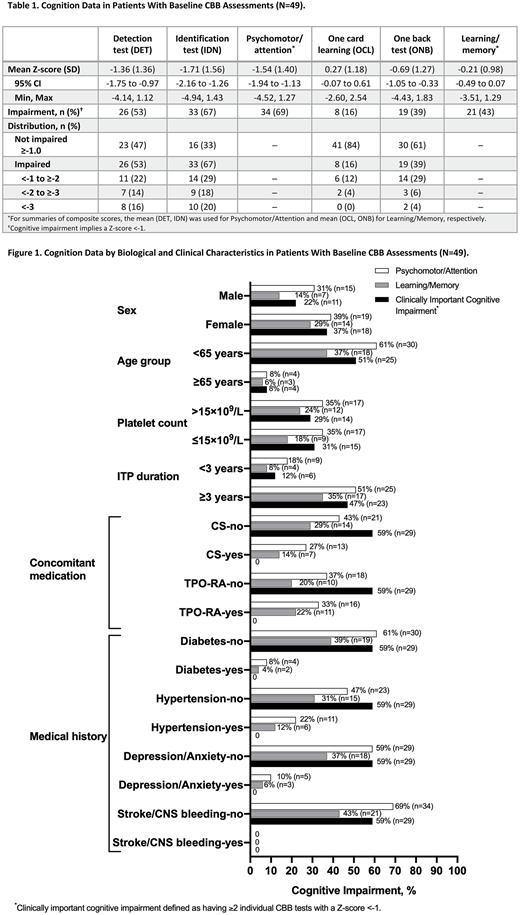
Results: At baseline, the 49 patients with CBB assessments had a mean age of 50 years, 57% were females, and 71% had an ITP duration of ≥3 years; 14 patients each had concomitant corticosteroids or thrombopoietin receptor agonists (TPO-RA) and 7 patients had both concomitant therapies. Medical history included hypertension (n=17), depression/anxiety (n=8), diabetes (n=7), and stroke/CNS bleeding (n=1). Mean Z-scores for the psychomotor function, attention, and working memory tests were below age-matched healthy norms (Table 1). The degree of impairment was substantial for the psychomotor function and attention tests and composite scores. Impairment was also large for the working memory test, whereas performance on the visual learning test was close to normal. The level of impairment followed the same pattern, with top rates for the attention (67% of patients), followed by psychomotor function (53% of patients), working memory (39% of patients), and visual learning (16% of patients) tests. Psychomotor/Attention impairment was greater (69% of patients) than Learning/Memory (43% of patients; Table 1) and occurred irrespective of biological/clinical characteristics (Figure 1). More than half of patients aged <65 years, with no concomitant ITP medication, and those with no medical history had a "Clinically Important Cognitive Impairment".
Conclusion: This report provides evidence of cognitive impairment in patients with ITP. The cognitive impairment was moderate in magnitude for Psychomotor/Attention, and mild for Learning/Memory. Further, the observed impairment appeared to be irrespective of biological/clinical characteristics and unrelated to concomitant use of corticosteroids or TPO-RA.
Disclosures
Kuter:Shire: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Platelet Disorder Support Association: Consultancy; Sanofi: Consultancy; Shionogi: Consultancy; Momenta: Consultancy; Merck Sharp & Dohme: Consultancy; Kyowa Kirin: Consultancy; Incyte: Consultancy; Genzyme: Consultancy; Dova: Consultancy; Daiichi Sankyo: Consultancy; CRICO: Consultancy; Caremark: Consultancy; BioCryst: Consultancy, Research Funding; Up-To-Date: Consultancy; BMS: Consultancy, Research Funding; Immunovant: Consultancy, Research Funding; Kezar: Research Funding; Principia: Consultancy, Research Funding; Protalex: Consultancy, Research Funding; Rigel: Consultancy, Research Funding; Takeda (Bioverativ): Consultancy, Research Funding; UCB: Consultancy, Research Funding; Rubius: Current holder of stock options in a privately-held company; Zafgen: Consultancy; Platelet Biogenesis: Consultancy; Amgen: Consultancy, Research Funding; argenx: Consultancy, Research Funding; Alnylam: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Actelion (Syntimmune): Consultancy, Research Funding; Cellularity: Consultancy; Cellphire: Consultancy; Hengrui: Consultancy. Khan:Seattle Children's Hospital: Ended employment in the past 24 months; Sanofi: Current Employment, Current equity holder in private company. Maruff:Cogstate Ltd: Current Employment. Edgar:Cogstate: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Cromer:Cogstate Ltd: Current Employment, Current equity holder in publicly-traded company. Stellman:Cogstate Ltd: Current Employment, Current equity holder in publicly-traded company. Daak:Sanofi: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal